Abstract
Introduction The development of inhibitors is an extremely rare complication in patients with factor (F)VII deficiency treated with replacement therapies such as recombinant activated FVII (rFVIIa), and represents a major therapeutic challenge in the treatment of this disorder. When inhibitors result in loss of response to high doses of rFVIIa, activated prothrombin complex concentrate (aPCC) is often used as an alternative treatment for these patients. The use of high-dose rFVIIa or aPCC might be accompanied by an increased risk of thromboembolic events, so it is important monitoring properly the effect of the hemostatic treatment
Here we describe the therapeutic management and follow-up of a patient with elevated inhibitor titres, resistant to rFVIIa treatment who switched to prophylaxis with aPCC, using several global coagulation tests.
Methods The coagulation status of the patient and the reponse to in vitro spiking of different doses of rFVIIa or aPCC were assessed with several global tests: Prothrombine time (PT) was tested using HemosIL®RecombiPlasTin 2G.
Clot formation was evaluated with Rotational Thromboelastometry (ROTEM®) using blood with CTI (to inhibit contact activation) and activated with a low amount of Tissue Factor (TF) as trigger (dilution 1:50,000 of EXTEM reagent).
The thrombin generation (TG) was tested with the Calibrated Automated Thrombogram (CAT) system using plasma collected with CTI and activated with 1 pM TF plus phospholipids (PPP-Low®, Stago).
Thrombus formation under flow conditions was assessed with the Total Thrombus-formation Analysis System (T-TAS®) in AR-chips coated with collagen and thromboplastin. The Area under the flow pressure curve (AUC) was calculated over 30 min after starting.
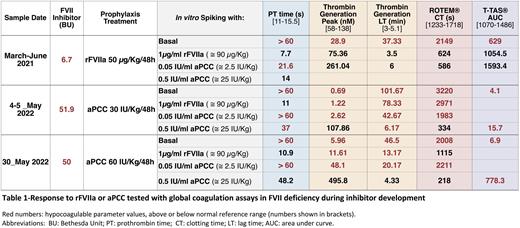
Results Three year-old girl affected by severe congenital FVII deficiency (FVII 9.1%) with significant severe bleeding history (spontaneous intracranial hemorrhage at 1st month of life). Since then she is in prophylaxis treatment with rFVIIa. At 6 months of life she developed an inhibitor (9.8 BU). Despite prophylaxis she presents frequent spontaneous mucocutaneous bleedings. When she was 2 years old she needed to be admitted at hospital because of port-A-Cath infection. Due to the administration of intensive treatment with rFVIIa during admission she developed thrombosis of peripheral venous access, so prophylaxis treatment was temporary suspended. After 5 days without prophylaxis treatment, and increasing inhibitor titres, she developed a new intracranial haemorrhage, which needed intensive treatment with continuous rFVIIa infusion (90 µg/Kg/12h), immunoglobulines and rituximab for lowering inhibitor titre. Hemostatic parameters improved during the administration of these treatments and inhibitor level was reduced. After restarting prophylaxis treatment with rFVIIa (50 µg/Kg/48h), inhibitors and a hypocoagulable state were observed with all global coagulation test showing extended PT, clotting time (CT), TG Lag-time (LT) and anomalous thrombus formation (AUC) but presented very good response to low dosis (2.5 IU/Kg) of aPCC in vitro (Table-1).
At the age of three years she required admission at hospital because of port-A-Cath infection again, needing its replacement by a new one after administration of high doses of rFVIIa without bleeding complications. After this, inhibitor titre raised again (> 50 BU), presenting frequent bleeding episodes (spontaneous muscle bleedings and tonsillar bleeding), and due to the clinical worsening, treatment was modified to prophylaxis with aPCC (30 UI/Kg/48h). Despite the treatment change she continued presenting bleeding episodes, global tests continued showing an hypocoagulable state, and required 10 times higher doses in vitro of aPCC (25 IU/Kg) to restore all the monitored hemostatic parameters (Table-1), probably related to the important increase in inhibitor titre. This situation led to employing higher doses of aPCC prophylaxis (60 UI/Kg/48 h) to try to avoid bleeding episodes without increasing the risk of suffering thrombotic events.
Conclusions Global coagulation tests used in this study were sufficiently sensitive methods to show an abnormal hemostatic profile in FVII-deficient patients with clinical hemorrhagic symptoms with inhibitor development, supporting their use to manage the risk of bleeding or thrombosis in this life-threatening condition.
Disclosures
Alvarez Román:Novo Nordisk: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Sponsored Symposia; Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Sponsored Symposia; Roche: Membership on an entity's Board of Directors or advisory committees, Other: Sponsored Symposia; Pfizer: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Sponsored Symposia; Octapharma: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Sponsored Symposia; Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Sponsored Symposia; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Sponsored Symposia; CSL-Behring: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Sponsored Symposia; Sobi: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Advisory Board ; Biomarin: Consultancy, Honoraria, Research Funding; Bayer: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other; Grifols: Consultancy, Honoraria, Research Funding. Butta:CSL-Bering: Research Funding; Novo-Nordisk: Speakers Bureau; Takeda: Research Funding, Speakers Bureau; Roche: Speakers Bureau. Hermans:oche, Novo Nordisk, Sanofi, Sobi, Takeda, Grifols, Bayer, Pfizer, Spark, BioMarin, Octapharma, CSL Behring: Consultancy; oche, Novo Nordisk, Sanofi, Sobi, Takeda, Grifols, Bayer, Pfizer, Spark, Octapharma, CSL Behring: Research Funding; oche, Novo Nordisk, Sanofi, Sobi, Takeda, Grifols, Bayer, Pfizer, Spark, Octapharma, CSL Behring: Honoraria. Jiménez-Yuste:Roche, Novo Nordisk, Sanofi, Sobi, Takeda, Grifols, Bayer, Pfizer, Spark, BioMarin, Octapharma, CSL Behring: Consultancy; Roche, NovoNordisk, Sanofi, Sobi, Takeda, Grifols, Bayer, Pfizer, Octapharma, CSL Behring: Research Funding; Roche, NovoNordisk, Sanofi, Sobi, Takeda, Grifols, Bayer, Pfizer, Spark, Octapharma, CSL Behring: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal